Patient Update – Important FDA Recommendation!
If you or a loved one have an implanted inferior vena cava (IVC) filter, it is critical you know the facts. An FDA-supported study has found that “the risks of complications start to outweigh the protective benefits of the filter at day 35 post-implantation.” The FDA now recommends IVC filters be removed between 29 and 54 days after implantation in the interest of patient safety.
Call Us toll-free at 1-800-LAW-5432 for a Free Case Review
Do Not Wait For Injuries To Happen. Physicians working with Marc J. Bern & Partners attorneys encourage all patients with an IVC filter implanted more than 3 months from today to immediately inform their medical providers if they have not had a post-implant evaluation.
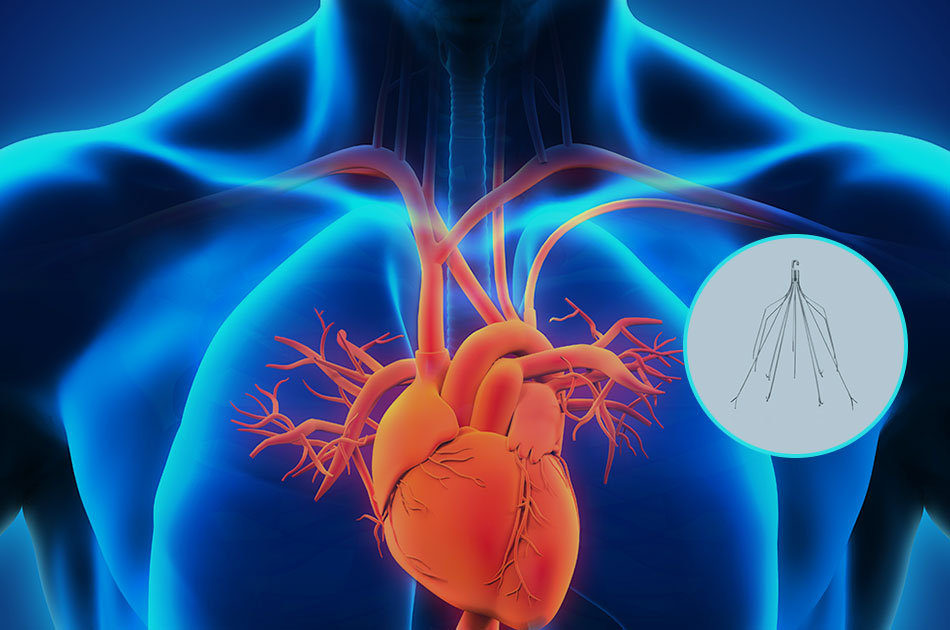
What is an IVC Filter?
An IVC filter is a tiny cone-shaped device implanted just below the kidneys. The IVC is the largest vein in the body, responsible for carrying de-oxygenated blood from the lower extremities to the right atrium of the heart and then to our lungs.
The IVC filter is designed to capture blood clots that have broken loose from one of the deep veins in the legs before reaching our heart and lungs. By permitting blood to travel around the clot, the IVC filter provides potentially life-saving time for our bodies’ natural anticoagulants to help break down the clot.
Patients at risk of developing blood clots – often due to unrelated surgical procedures – but who are not candidates for blood thinner medication, often have IVC filters implanted to reduce the risk of artery blockage (often referred to as a pulmonary embolism) resulting in breathing difficulties, chest pain, and death.
Why is there IVC Filter Litigation?
According to published reports, approximately 200,000 blood clot filters are implanted every year. In 2015 alone, the market for these products was expected to reach $435 million.
However, in 2010, the Archives of Internal Medicine published a study which found that only half of all IVC filter implantation surgeries were medically necessary. The study analyzed 1,547 patients diagnosed with acute venous thromboembolism (VTE) who had IVC filters implanted and found that 25% of those patients did not need the filter. Researchers were not able to reach a consensus regarding the 23% percent of patients, and found that IVC filter patients had more than double the higher in-hospital mortality rate.
Shortly after the Archives of Internal Medicine study was published, the FDA announced that 921 adverse event reports had been filed. Of the 921 adverse event reports, there were 328 instances of filter migration, 146 device detachments, 70 inferior vena cava perforations, and 56 filter fractures.
That same year, 2010, the FDA expressed concern that retrievable IVC filters were being left in patients for too long and recommended surgeons remove the devices as soon as possible. Four years later, in 2014, the FDA reiterated its position in the face of mounting evidence that retrievable IVC filters – which are intended for short-term placement – were not being removed within the appropriate time periods.
In 2013, a study published in JAMA Internal Medicine found that “approximately 91.5% of retrievable filters placed in patients at risk for VTE became permanent filters,” despite the fact that “recent data show that increasing complications occur when the filters are left in place for longer periods.”
Who may be Responsible?
In July 2015, C.R. Bard received a warning letter from the FDA identifying eight violations of federal law associated with its IVC filters.
Tellingly, this FDA warning letter came more than 10 years after C.R. Bard – the leading manufacturer of IVC filters – began receiving a significant number of complaints of post-implantation loosening, fracturing and migration of its IVC filters.
However, instead of properly communicating these reports to the FDA back in 2004, it is alleged that Bard merely compared newer models to older models in an effort to tout improved failure rates. And the public only knows about this alleged cover-up because Bard’s mistakenly released an internal document detailing its conduct. This document suggests that Bard failed to warn patients, doctors, and the public of known risks associated with its IVC filter products.
Medical studies indicate failure rate of just one C.R. Bard IVC filter product – the Bard Recovery® — is between 21% and 31.7%:
- A 2005 study by the New England Society for Vascular Surgery found a 31.7% Bard Recovery® fracture rate after examining adverse event reports filed with the FDA.
- A 2008 study in the Journal of Vascular and Interventional Radiology indicated a 21% Bard Recovery® failure rate among patients included in the study.
- A 2010 study in the Archives of Internal Medicine found that the rate of complications with Bard Recovery® IVC filters was 25%, with researchers finding that pieces of the Bard Recovery® filter migrated to the heart in more than 70% of those who experienced a fracture.
What You Need to Know
The following IVC filters – manufactured by C.R. Bard and Cook Medical – have been alleged to cause serious injuries in patients:
- Recovery®
- G2®
- G2 Express® (G2X®)
- Eclipse®
- Meridian®
- Denali® Vena Cava
- Simon Nitinol®
- Recovery Cone Removal Kit®
- Celect®
- Gunther Tulip®
Marc J. Bern & Partners’ medical device litigation team are providing free consultations and claim evaluations to all individuals who received an IVC filter since 2003. All cases are pursued on a contingency fee basis, which means that there are no fees or expenses associated with this litigation unless we win your case.